Hormones, insulin and blood sugar regulation
This subpage is the fourth part of the theory of Biotech Academy’s material on Diabetes.
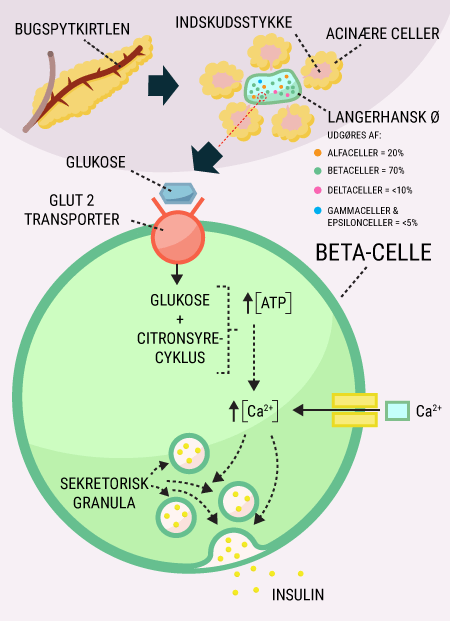
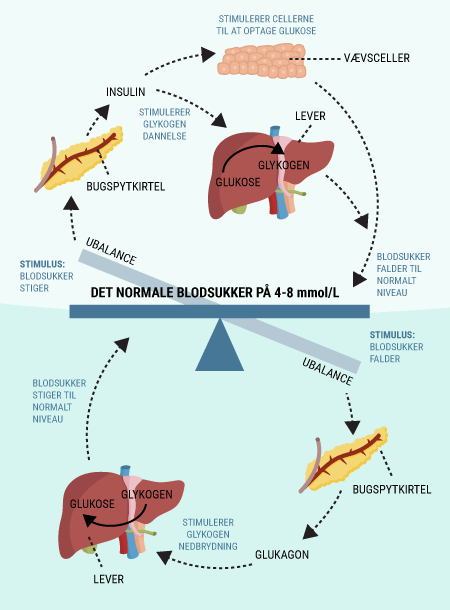
You have now been introduced to some of the important organs associated with diabetes, including the digestive tract and pancreas. The pancreas, which produces and releases the hormones glucagon and insulin, among others, plays a major role in keeping blood sugar levels more or less constant. You have also read about the function of glucose in the body and how it is stored, broken down and used by the body’s cells. This section explains why many of these processes are dependent on insulin.
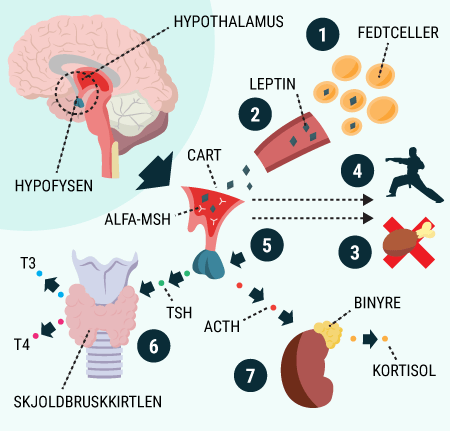
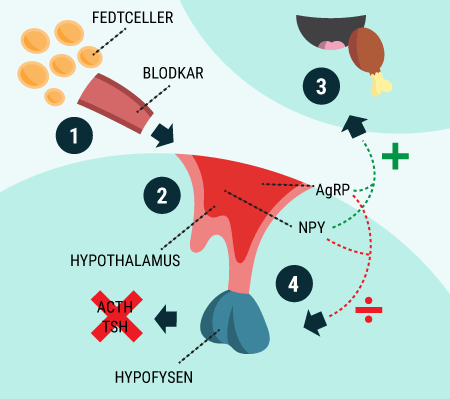
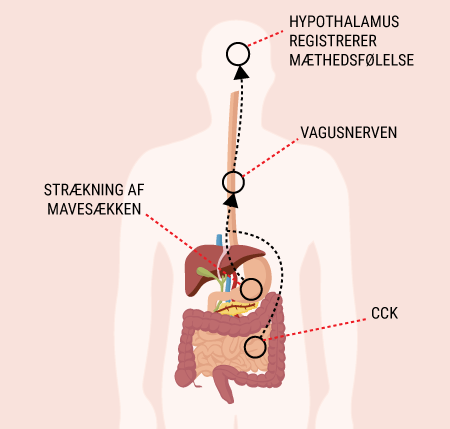
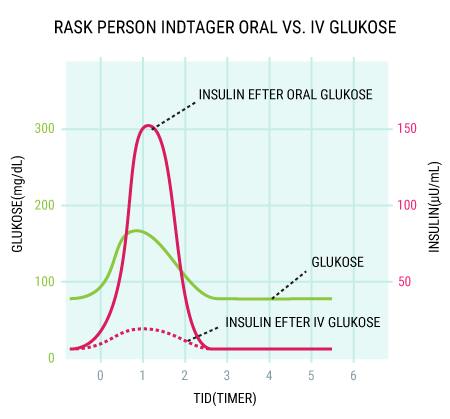
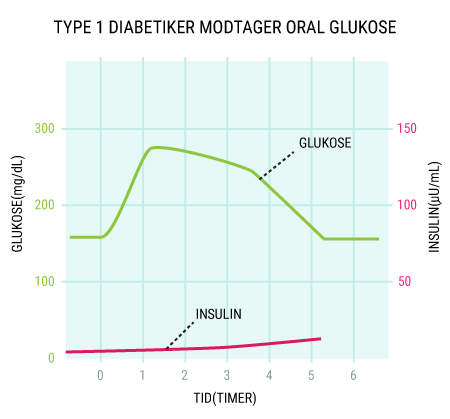
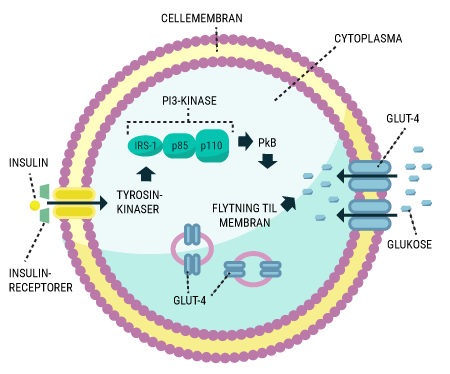
You will be able to read about how the different hormones described earlier affect each other and how together they regulate the body’s blood sugar levels and thus sustain our lives. You probably already know that even a short-term lack of oxygen to the brain will cause serious brain damage or even death, but did you know that the brain’s need for food in the form of glucose is just as important? Even a few minutes of glucose deprivation will cause unconsciousness and then death if glucose is not administered in time. After reading this article, you will know how our appetite is regulated and you will have learned about the key hormones related to satiety. You will get an explanation of how and when insulin is released and how blood sugar is regulated in a healthy individual. And last but not least, you want to know how insulin works when it is released into the bloodstream.