CRISPR/Cas9 – The genetic engineering revolution
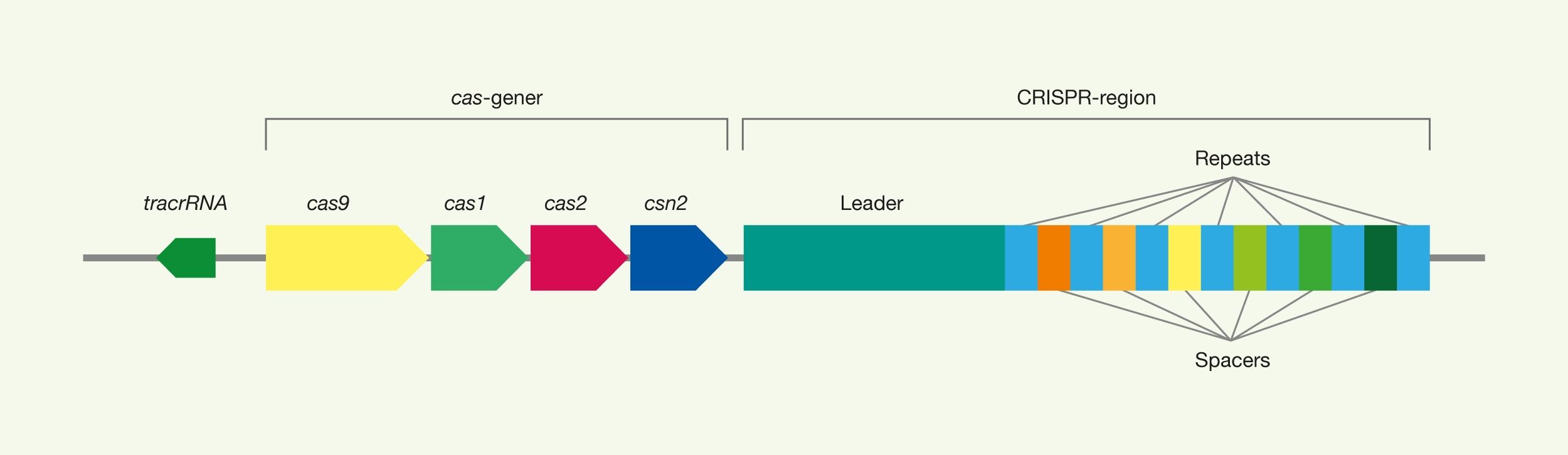
Summary: CRISPR technology comes from the immune system of prokaryotes, where it acts as protection against viral attacks, for example. CRISPR is an abbreviation of Clustered Regularly Interspaced Short Palindromic Repeats, which describes the genomic structure of the prokaryotic immune system. CRISPR associated (Cas) proteins are the functional units of the immune system. The most commonly used type of Cas9 in genetic engineering comes from the bacterium Streptococcus pyogenes.
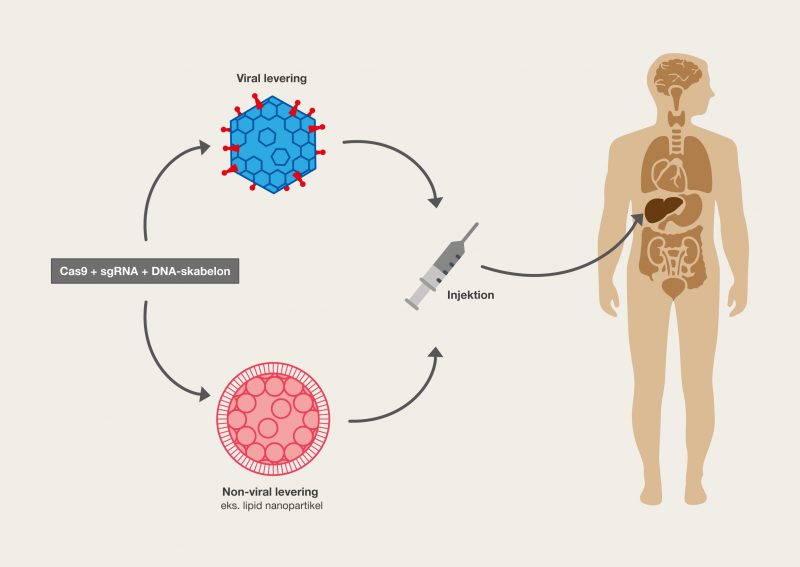
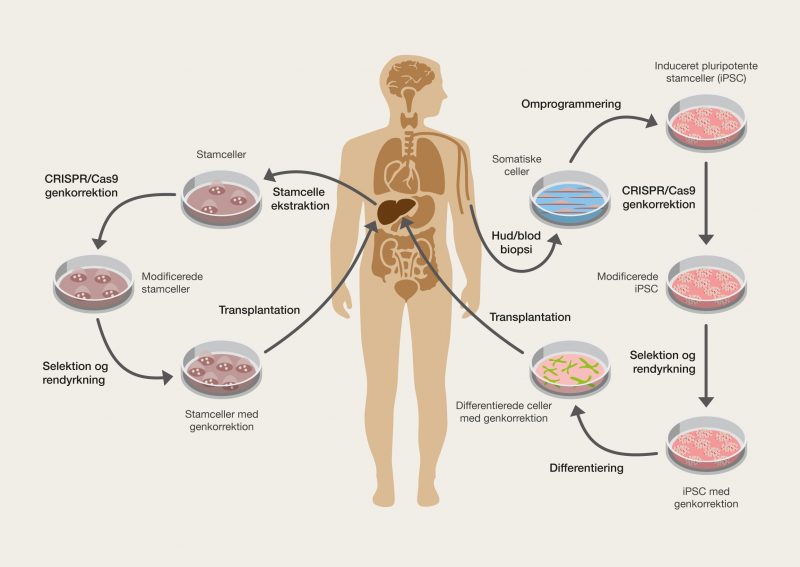
Everything that can be defined as life has one unique thing in common. All the information that forms individual organisms is chemically stored in a macromolecule that functions in the same fundamental way in man and all other organisms. DNA is the molecule that makes up our genetic information through its genetic code. It is of enormous importance that life shares the foundations of this genetic code, as it means that it is possible to take information from one organism and use it in another. Genetic engineering makes it possible to move genes around and control their activity, so that we can actually control the shape of life. Genetic engineering can be used to create untold amounts of genetic combinations that, for example, can give us better food, change the properties of organsims, be used for the production of medicine or even cure diseases. It is only the imagination and technology that limits the possibilities – fortunately, technology has come a long way!
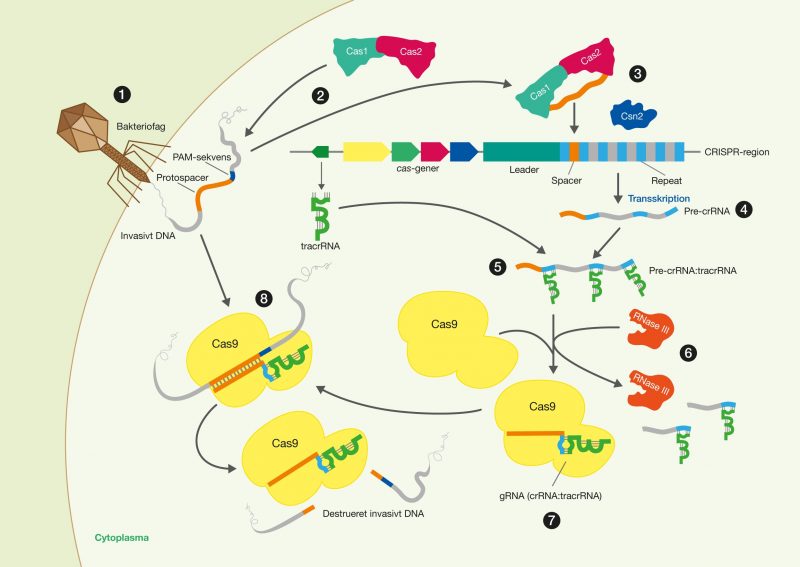
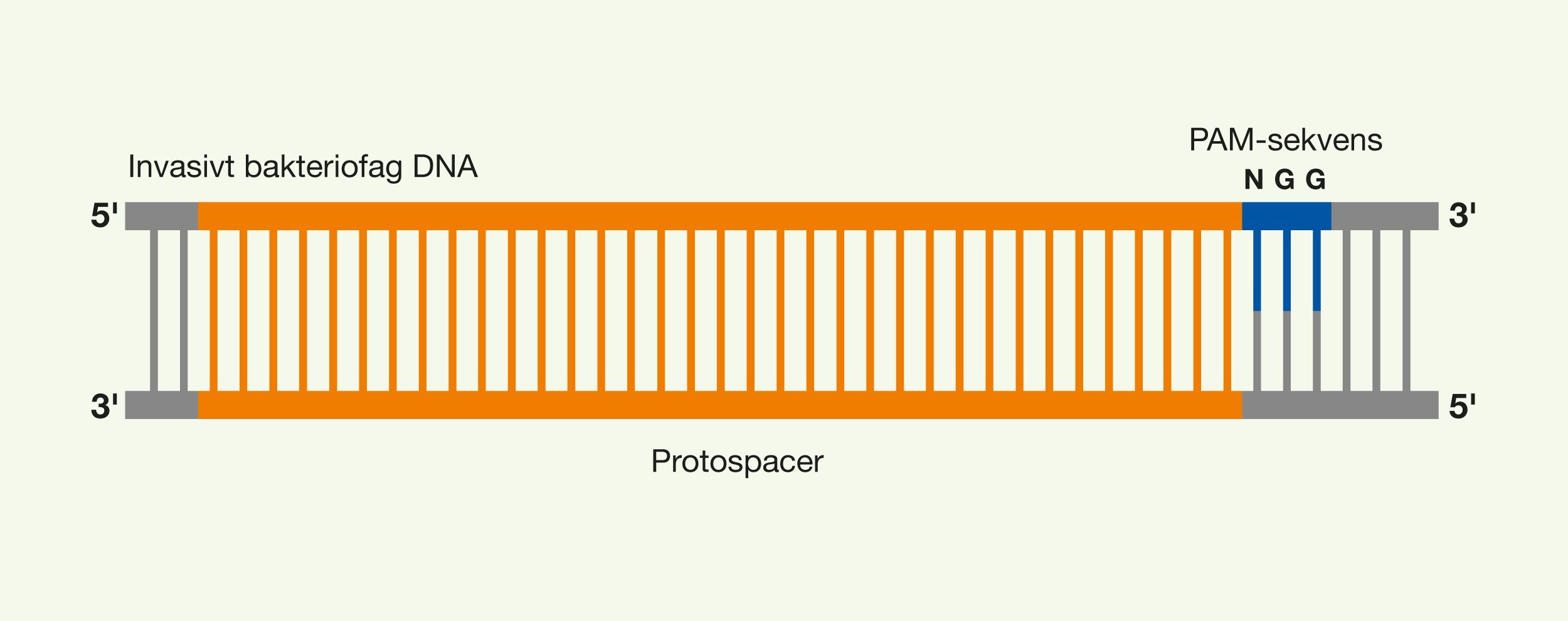
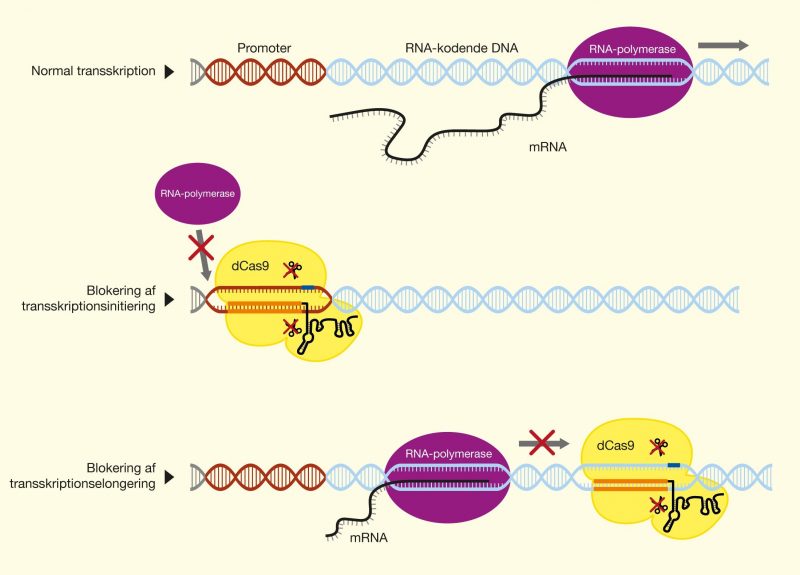
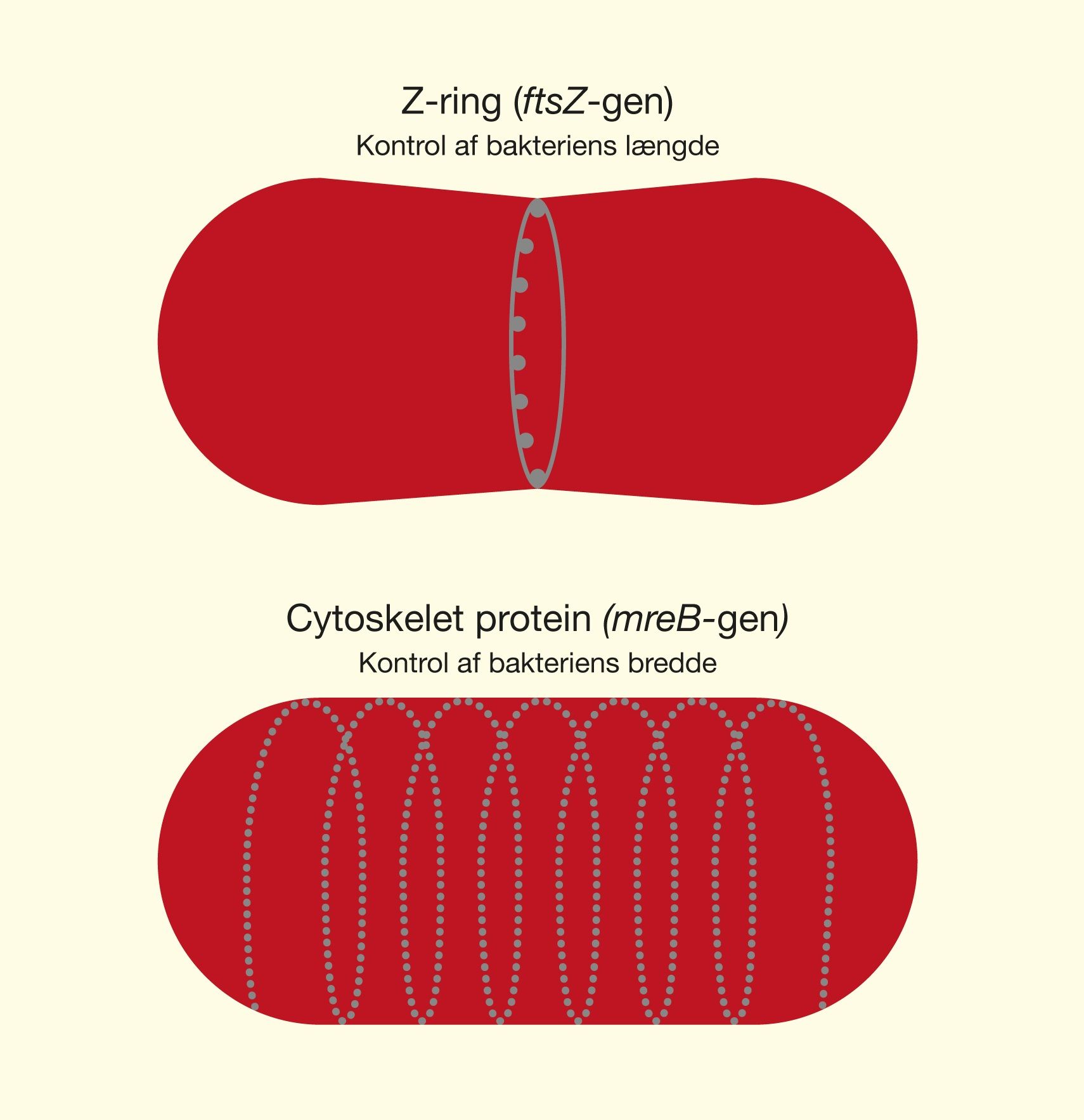
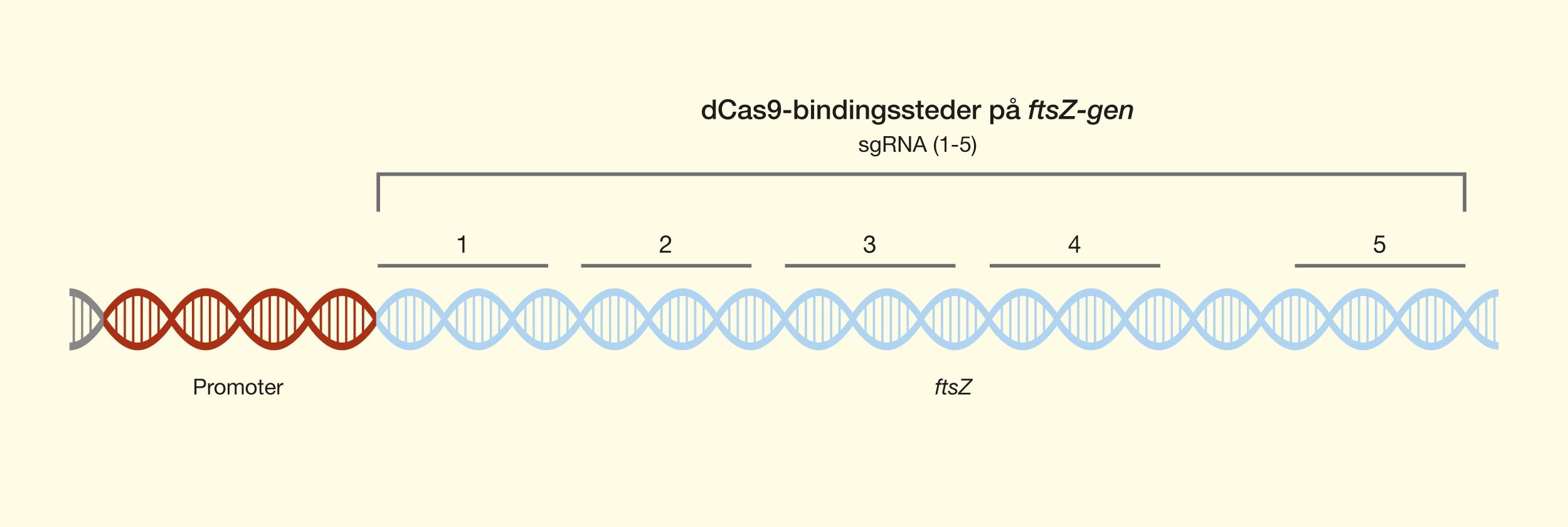
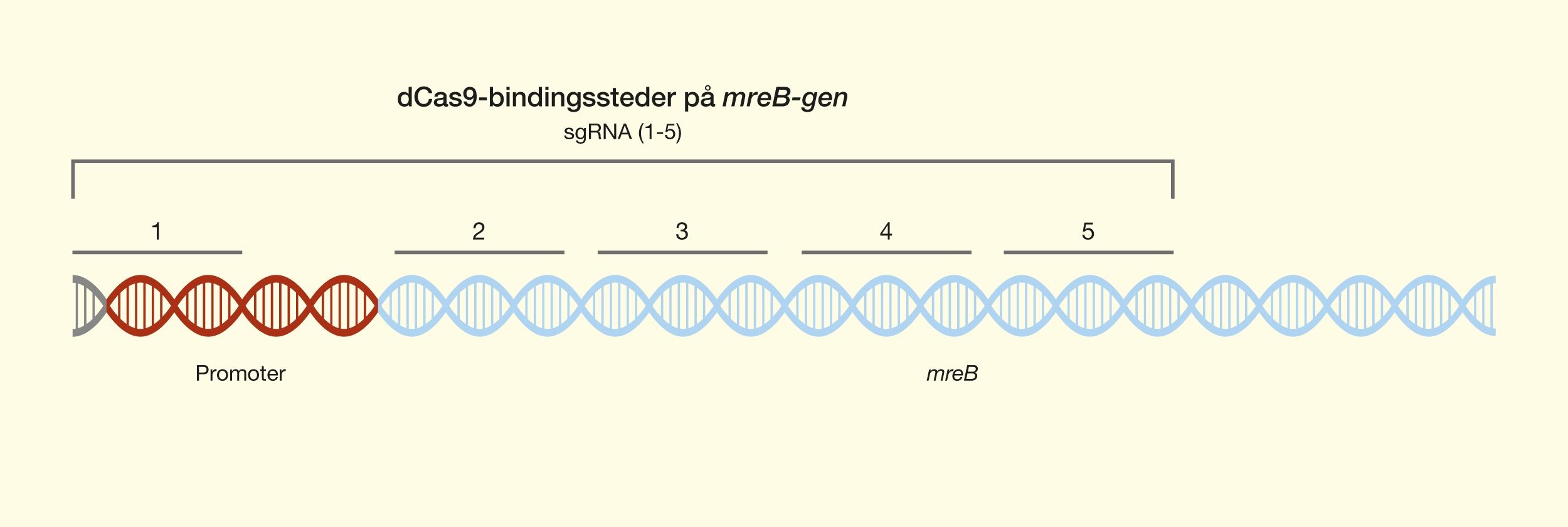
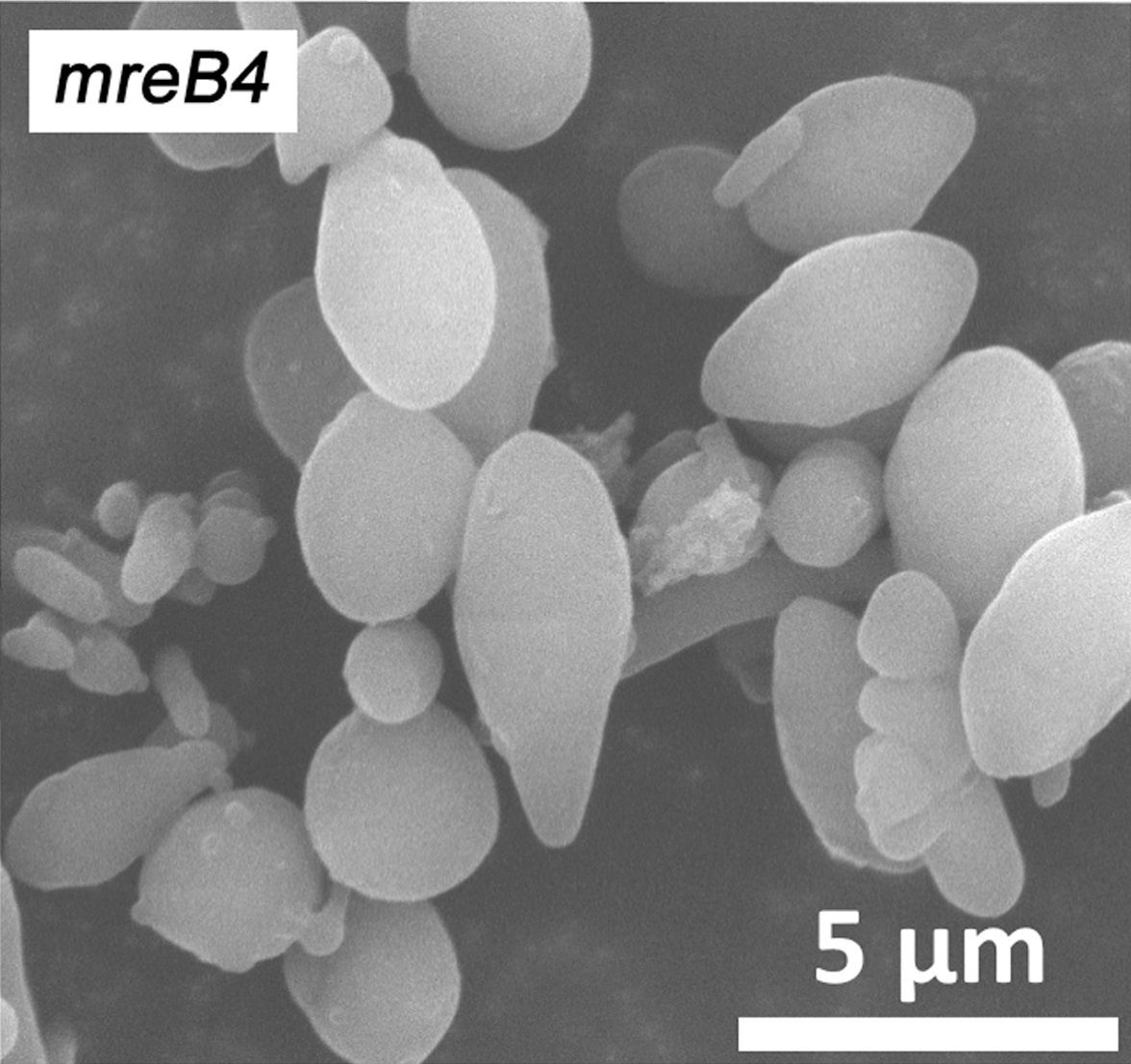
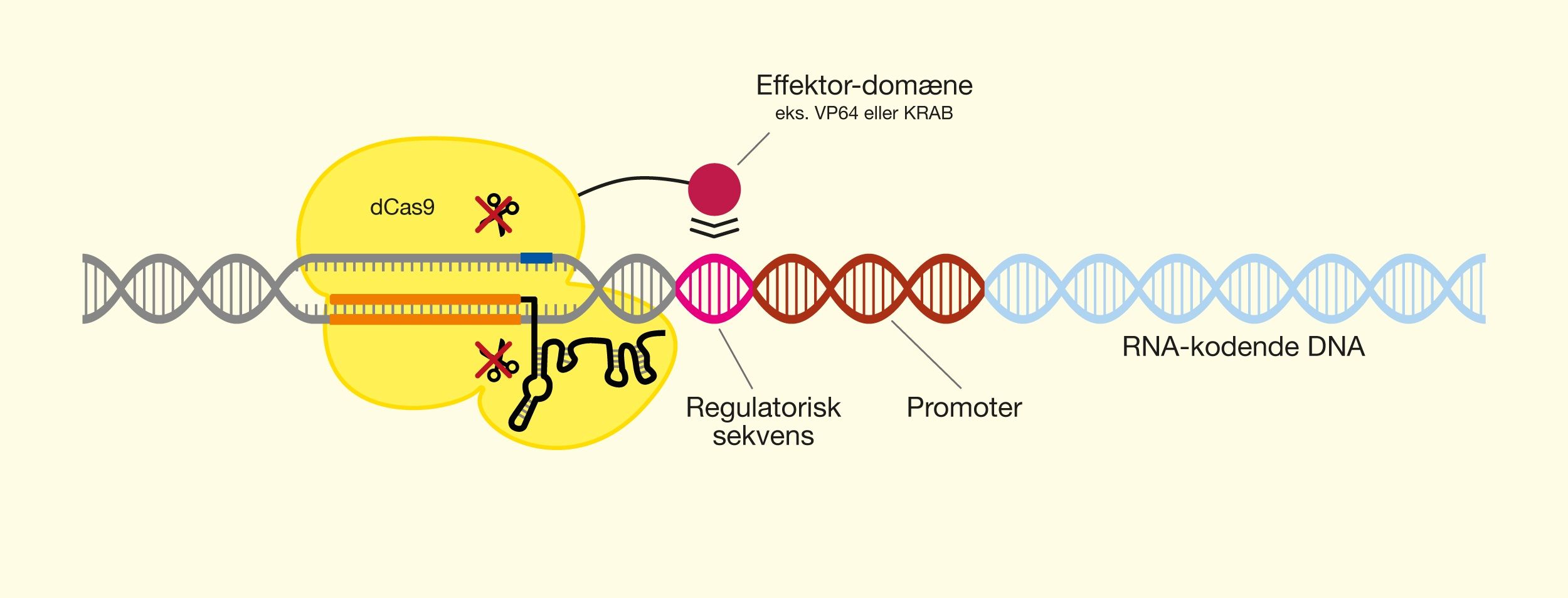
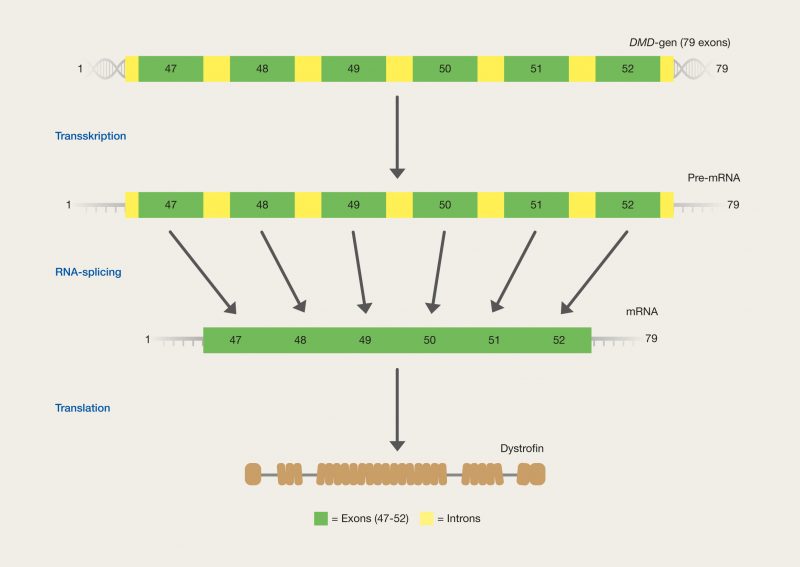
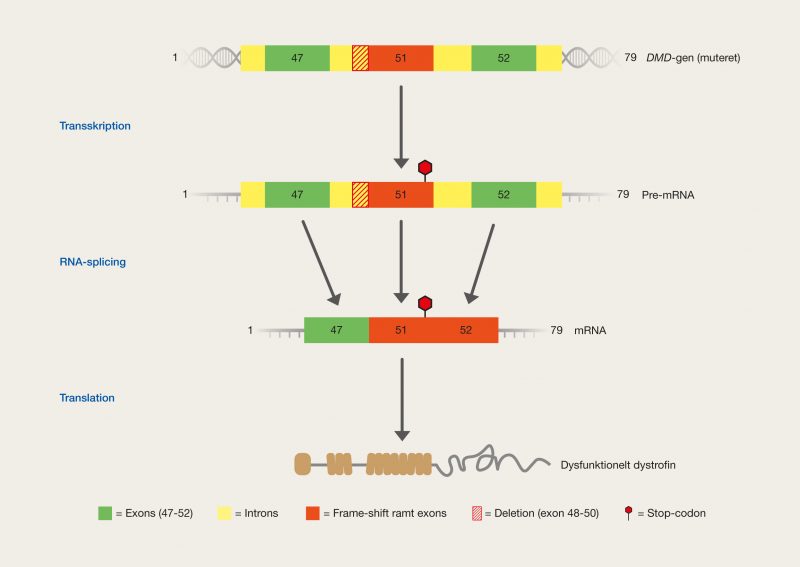
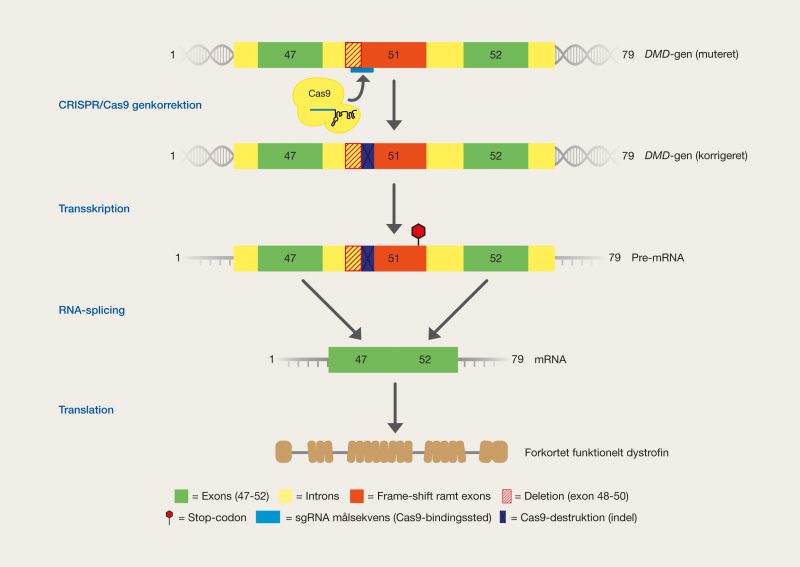
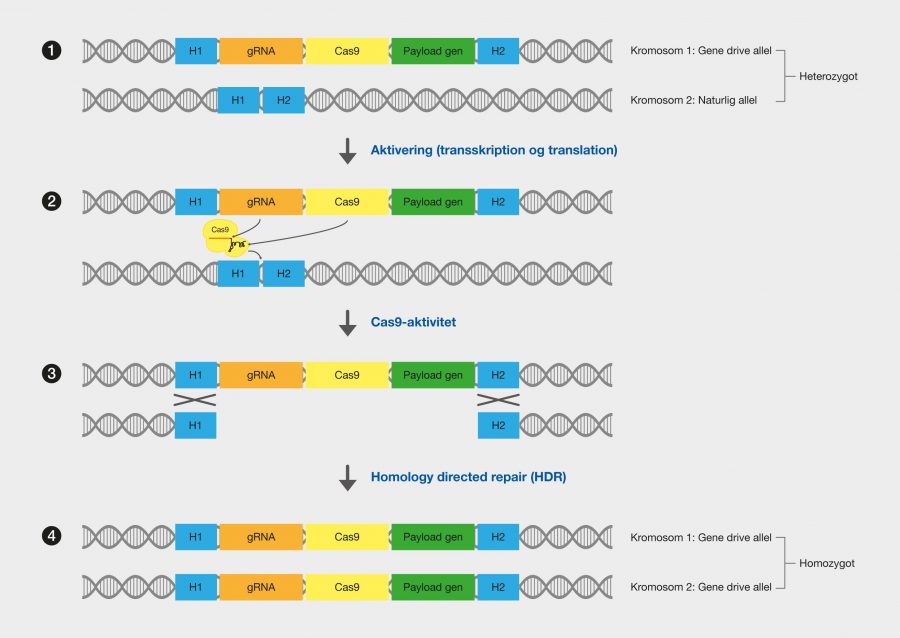
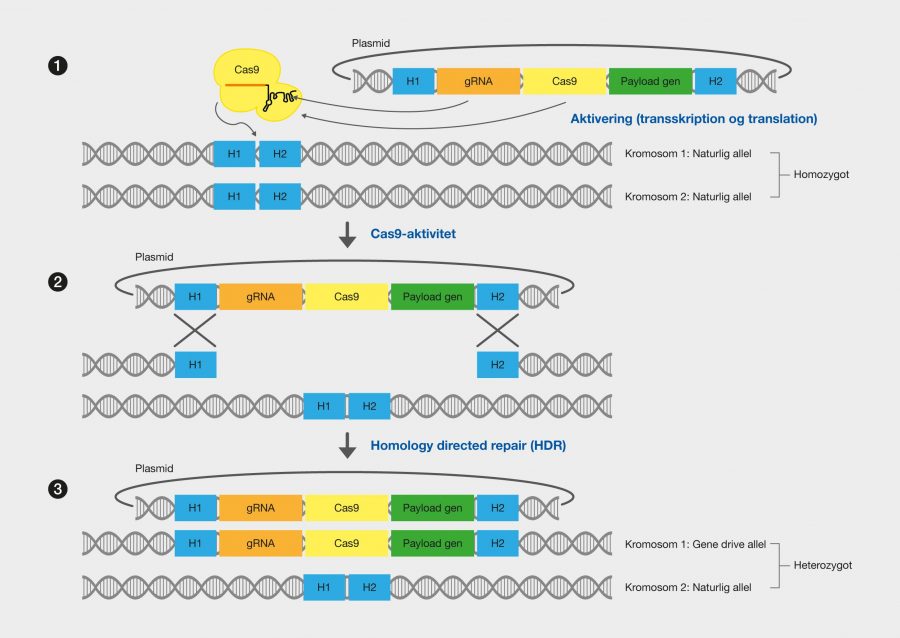
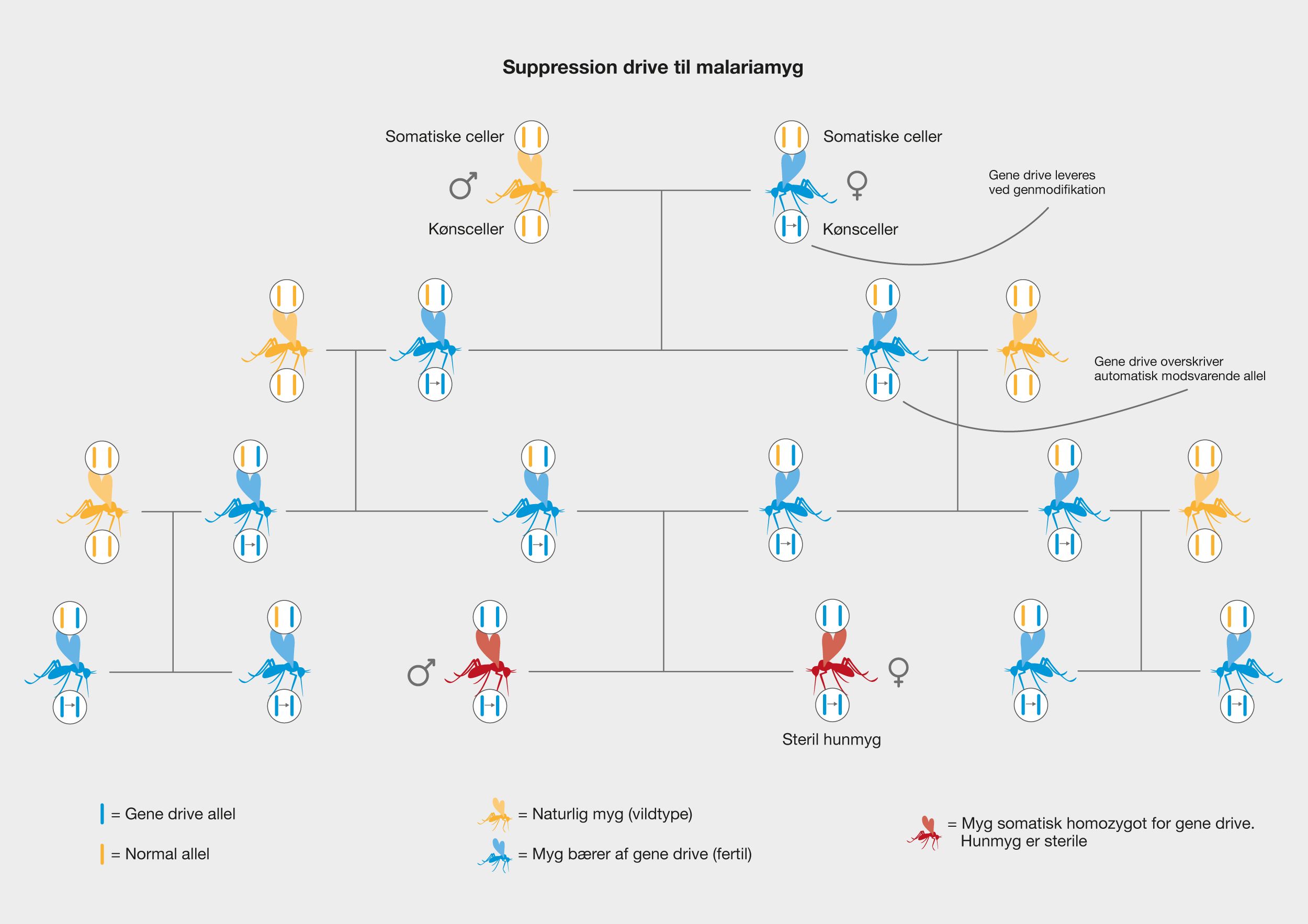
One of the latest technologies is CRISPR/Cas9, which has great potential because it provides the opportunity to cut DNA with enormous precision and efficiency, in an easier and cheaper way. This technology is based on one protein called Cas9. The protein is controlled by a piece of RNA called guide RNA (gRNA), which gives the protein its property to accurately identify DNA sequences, bind to them, and cut them by forming precise double-strand breaks in DNA. The reason why Cas9 has so much potential is that the protein can easily be reprogrammed to target new DNA sequences by replacing the gRNA that controls the protein. Cas9’s precise formation of double-strand fractures allows, for example, the insertion of DNA sequences into very specific positions with which new gene functions can be obtained. One can also form mutations that can deactivate specific genes and thus remove specific traits from organisms.
The CRISPR technology originates from the immune system of prokaryotes, where it acts as protection against viral attacks, for example. CRISPR is an abbreviation of C lustered Regularly Interspaced Short Palindromic Repeats, which describes the genomic structure of the prokaryotic immune system. CRISPR asassociated (Cas) proteins are the functional units of the immune system. The most commonly used type of Cas9 in genetic engineering comes from the bacterium Streptococcus pyogenes.
CRISPR/Cas9 is a magnificent example of a biotechnological tool developed from nature’s own functional mechanisms.
The basic theory will provide a good basic understanding of the molecular function of CRISPR/Cas9 and how we can exploit it for gene modification. Then the different cases can be read independently of each other.