Functional groups
Functional groups are specific groups of atoms in an organic molecule that have certain chemical characteristics. It is typically the functional groups in a molecule that react when a molecule enters a reaction. A functional group defines a substance class. The chemical compounds in a substance class will react similarly. From the different functional groups in an organic molecule, you can get an idea of which reactions the molecule can take part in.
Single, double and triple bonds in alkanes, alkenes and alkynes are also functional groups. Below is a brief overview of the functional groups that are essential for this learning material.
R is a term for side chains that are attached to the main chain of a molecule you are looking at. R is used in organic chemistry, which means that R groups must contain a carbon chain. R is often used if you only want to look at a smaller part of a larger molecule or, for example, only one functional group. If a molecule is shown with multiple R groups, the R groups can be the same or different. If the groups are different, they will typically be namedR1,R2,R3… etc.
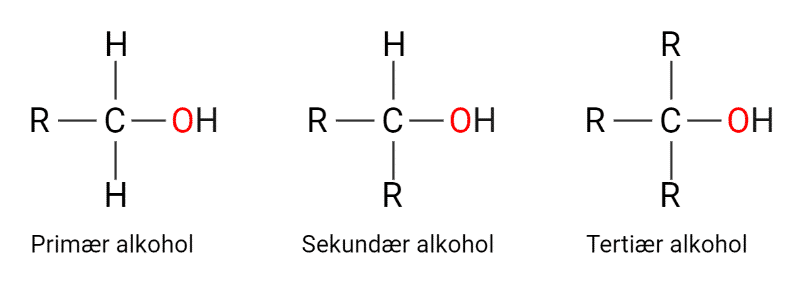
Hydroxy groups are -OH groups and are often called alcohol groups. Alcohols are classified as primary, secondary and tertiary alcohols. Classification is determined by how many hydrogen atoms are bound to the carbon atom to which the oxygen is bound.
Figure 4: Structure of primary, secondary and tertiary alcohol.
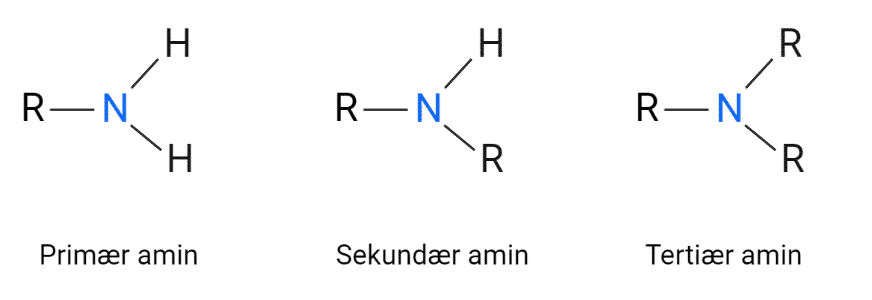
Amines are derivatives of ammonia (NH3) where either 1, 2 or all 3 hydrogen atoms are replaced by a side chain containing carbon. They are also classified as primary, secondary and tertiary amines. Amino acids in proteins contain a primary amine.
Figure 5: Structure of primary, secondary and tertiary amine.
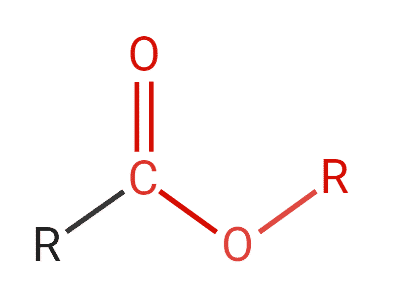
Esters are neutral compounds that are typically formed from a condensation reaction between a carboxylic acid and alcohol. Figure 6 shows the general structural formula for esters. They consist of a carbonyl, which is a carbon that is double bonded to an oxygen and an oxygen atom.
Figure 6: Structure of ester.
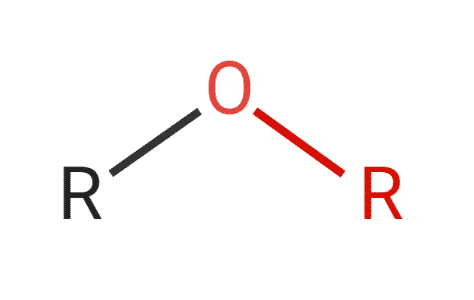
Ethereal consists of an oxygen atom bonded to two carbon atoms. Small ethers with short side groups are weakly polar. If the side chains are long, the ethers are non-polar.
Figure 7: Structure of ether.
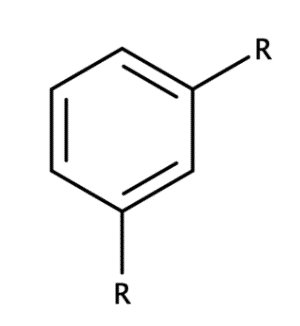
Aromatics are cyclic organic compounds that contain a benzene ring (C6H6) or are a cyclic compound (bonds that form a ring). The electronegativity between carbon and hydrogen is relatively small and there is no difference in electronegativity between carbon and carbon in a C-C bond. Therefore, these bonds are non-polar, making aromatic rings non-polar hydrophobic compounds. Figure 8 shows a benzene ring with two substituents attached.
Figure 8: Structure of benzene ring with two substituents.